Prof. Philipp Beckhove
Scientific Director LIT & Head of Research Division | Interventional Immunology

For over 25 years, Professor Philipp Beckhove has been dedicated to studying the interaction between the human immune system and cancer. In particular, he has looked at how T cells in patients respond spontaneously to recognize tumor cells and whether such responses impact tumor development. He has demonstrated that in most patients tumor-specific T cells are generated at high frequencies, and belong to two major subtypes. The first (type 1 T cells) possesses the capacity to combat tumor cells, while the other (regulatory T cells) protects tumor cells against an immune attack and supports tumor growth. The generation of both subtypes is orchestrated within the bone marrow and essentially intertwined—explaining why simple concepts of tumor immunotherapy are often not effective: And it is based on these findings that new therapeutic approaches to harness the immune system against cancer are being developed.
Feuerer, Nat. Med. 2001; Feuerer, Nat. Med. 2003; Xydia, Nature Communication 2021, 12, 1119; Ge, Cancer Immunol Res. 2019 Dec;7(12):1998-2012; Carretero, Nat Immunol. 2015 Jun;16(6):609-17; Reissfelder, J Clin Invest. 2015 Feb;125(2):739-51; Klug, Cancer Cell, 2013, dxdoi.org/10.1016/j.ccr2013.09.014; Bonertz, J.C.l. 2009; 119: 3311-21., Nummer,J.N.C. 2007; 99: 1188-99
Expression patterns of 224 immune resistance genes in different human cancers. Expression strength is illustrated by colour code (red: high, blue: low). Gene expression data are retrieved from TCGA data base.
Recent work
A key approach to improving the anti-cancer efficacy of tumor-specific T-cell responses lies in their direct communication with the tumor cells. Professor Beckhove discovered that even fully activated cytotoxic T cells attacking a tumor cell will in most cases not eliminate it. Instead tumor growth and resistance to treatment is often stimulated. This is because tumor cells have the capacity to convert the cytotoxic signals of T cells into tumor-promoting signaling cascades within the tumor cell.
Professor Beckhove has systematically identified tumor-associated immune resistance genes and signaling cascades that are responsible for this malicious act. Together with his team he is now developing inhibitors for these immune resistance genes, and genetically reprograming tumor-specific cytotoxic T cells so that they can overcome tumor-intrinsic immune resistance—and efficiently eradicate tumors (Witzens-Harig 2013, Blood;121(22):4493-503; Khandelwal, EMBO Mol Med. 2015 Feb 17;7(4):450-63; Volpin, Cancer Immunol Res 2020 Sep;8(9):1163-1179; Menevse, Acta Neuropathol Commun. 2023; 11(1):75).

Through Professor Beckhove’s work, an inhibitor of salt-inducible kinase 3—a major immune-resistance gene in pancreatic cancer—has been developed. This is now in clinical testing for the treatment of advanced metastasized cancer (Sorrentino, J.I.T.C. 2022; 10(5):e004258, Clinical Trials.gov Identifier: NCT05826600).
Quote from Professor Philipp Beckhove
Tumor cells can convert the immune cells’ cytotoxic signals to improve their own fitness and even boost tumor growth. By deciphering the landscape of tumor-intrinsic immune resistance genes we can develop new strategies to empower T cells so that they overcome these hurdles, and can efficiently eradicate even difficult-to-treat cancers.
Scientific Director LIT & Head of Research Division Interventional Immunology
Biography

Academic background and qualifications
Prof. Beckhove graduated in medicine from the University of Heidelberg in 1996, and completed his residency at the University Medical Center of Heidelberg achieving the Board Certificate in Internal Medicine in 2009. He received postdoctoral training in Cellular Immunotherapy and Tumor Immunology alongside Prof. Schirrmacher at the German Cancer Research Center (DKFZ), Heidelberg from 2001–2006.

Professional career
Prof. Beckhove remained at the DKFZ as an independent junior group head (2006–2008), head of an independent DKFZ research unit (2008–2011), and finally as Head of the DKFZ research division for Translational Immunology (2011–2015). Since 2015, he has been Professor (Chair) for Interventional Immunology at the University of Regensburg’s medical faculty, and additionally occupied the post of Scientific Director at the Regensburg Center for Interventional Immunology from 2015–2021. Since 2022 he has been Scientific Director at the Leibniz Institute for Immunotherapy.

Honors
Prof. Beckhove has published more than 200 papers and has been the recipient of several prizes and honors that include; the W.G. Forbeck Scholar Award 2000; the Sir Hans Krebs Award 2001; the Walther und Christine Richtzenhain Award 2003, and the Clinical Science Award 2004 from the Swiss Society for Immunotherapy. Since 2009 he has been Adjunct Associate Professor, Northwestern University, Chicago Ill. in the United States. Prof. Beckhove is the Chairman of the ‘Strategic Networks’ working group for the National Strategy for Gene and Cell Therapy at the Berlin Institute of Health. He is also Chairman of the Scientific Advisory Board at the Leibniz Institute for Natural Product Research and Infection Biology, HKI, and Co-founder and Chairman of the Scientific Advisory Board of iOmx therapeutics AG. He additionally acts as a scientific advisor for the German Cancer Aid Foundation (panel member for Cancer Therapy Studies), the Interdisciplinary Center for Clinical Research at the University of Münster (SAB member), the Federal State-funded scientific collaboration in cancer research between Israel and Germany (SAB member), and lastly, on the European Research Council (ERC panel member Immunology 2024/2025).
Explore our Research Division in greater depth
Get to know our team and find out more about our pioneering research.
Visit the complete publications list on Google Scholar:
https://scholar.google.com/citations?hl=en&hl=en&user=A_CVdsEAAAAJ
Here is a selection of the most important publications from the last few years:
- Xydia M, Rahbari R, Ruggiero E, Macaulay I, Tarabichi M, Lohmayer R, Wilkening S, Michels T, Brown D, Vanuytven S, Mastitskaya S, Laidlaw S, Grabe N, Pritsch M, Fronza R, Hexel K, Schmitt S, Müller-Steinhardt M, Halama N, Domschke C, Schmidt M, von Kalle C, Schütz F, Voet T, Beckhove P. Common clonal origin of conventional T cells and induced regulatory T cells in breast cancer patients. Nat Commun. 2021 Feb 18;12(1):1119. doi: 10.1038/s41467-021-21297-y. PMID: 33602930
- Khandelwal N, Breinig M, Speck T, Michels T, Kreutzer C, Sorrentino A, Sharma AK, Umansky L, Conrad H, Poschke I, Offringa R, König R, Bernhard H, Machlenkin A, Boutros M, Beckhove P. A high-throughput RNAi screen for detection of immune-checkpoint molecules that mediate tumor resistance to cytotoxic T lymphocytes. EMBO Mol Med. 2015 Apr;7(4):450-63. doi: 10.15252/emmm.201404414. PMID: 25691366
- Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M, Benner A, Büchler MW, Jäger D, Halama N, Khazaie K, Weitz J, Beckhove P. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015 Feb;125(2):739-51. doi: 10.1172/JCI74894. Epub 2014 Dec 22. PMID: 25562322
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schäkel K, Garbi N, Jäger D, Weitz J, Schmitz-Winnenthal H, Hämmerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013 Nov 11;24(5):589-602. doi: 10.1016/j.ccr.2013.09.014. Epub 2013 Oct 24. PMID: 24209604
- Witzens-Harig M, Hose D, Jünger S, Pfirschke C, Khandelwal N, Umansky L, Seckinger A, Conrad H, Brackertz B, Rème T, Gueckel B, Meißner T, Hundemer M, Ho AD, Rossi JF, Neben K, Bernhard H, Goldschmidt H, Klein B, Beckhove P. Tumor cells in multiple myeloma patients inhibit myeloma-reactive T cells through carcinoembryonic antigen-related cell adhesion molecule-6. Blood. 2013 May 30;121(22):4493-503. doi: 10.1182/blood-2012-05-429415. Epub 2013 Apr 19. PMID: 23603913
- Beckhove P, Warta R, Lemke B, Stoycheva D, Momburg F, Schnölzer M, Warnken U, Schmitz-Winnenthal H, Ahmadi R, Dyckhoff G, Bucur M, Jünger S, Schueler T, Lennerz V, Woelfel T, Unterberg A, Herold-Mende C. Rapid T cell-based identification of human tumor tissue antigens by automated two-dimensional protein fractionation. J Clin Invest. 2010 Jun;120(6):2230-42. doi: 10.1172/JCI37646. Epub 2010 May 10. PMID: 20458140
- Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009 Nov;119(11):3311-21. doi: 10.1172/JCI39608. Epub 2009 Oct 5. PMID: 19809157
- Nummer D, Suri-Payer E, Schmitz-Winnenthal H, Bonertz A, Galindo L, Antolovich D, Koch M, Büchler M, Weitz J, Schirrmacher V, Beckhove P. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst. 2007 Aug 1;99(15):1188-99. doi: 10.1093/jnci/djm064. Epub 2007 Jul 24. PMID: 17652277
- Choi C, Witzens M, Bucur M, Feuerer M, Sommerfeldt N, Trojan A, Ho A, Schirrmacher V, Goldschmidt H, Beckhove P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood. 2005 Mar 1;105(5):2132-4. doi: 10.1182/blood-2004-01-0366. Epub 2004 Nov 23. PMID: 15561890
- Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004 Jul;114(1):67-76. doi: 10.1172/JCI20278. PMID: 15232613
- Feuerer M*, Beckhove P*, Garbi N*, Mahnke Y, Limmer A, Hommel M, Hämmerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003 Sep;9(9):1151-7. doi: 10.1038/nm914. Epub 2003 Aug 10. PMID: 12910264 *; equal contributions
- Feuerer M*, Beckhove P*, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001 Apr;7(4):452-8. doi: 10.1038/86523. PMID: 11283672 *; equal contributions
Many thanks to the funding agencies who support our work:
Federal Ministry for Education and Research (BMBF) – SATURN3
‘Spatial and Temporal Resolution of Intratumoral Heterogeneity in Three Hard-To-Treat Cancers’
German Research Association (DFG)
Clinical Research Consortium FOR 2858: ‘The role of TSPO in T-Cell Immune Control of Glioblastoma’
German Cancer Aid
T-Lock Consortium to unravel immune regulatory pathways in malignant melanoma, NSCLC and colorectal cancer
European Union EU-ITN – PAVE
‘A Nanovaccine Approach for the Treatment of Pancreatic Cancer’
We participate in several national and international research consortia. These include:
SATURN3
This national research consortium to address ‘Spatial and Temporal Resolution of Intratumoral Heterogeneity in Three Hard-to-Treat Cancers’ is funded by the Federal Ministry of Education and Research (BmBF).
Clinical Research Consortium FOR 2858, SP A03
This consortium addresses ‘The Role of TSPO in T-Cell Immune Control Of Glioblastoma’ and is funded by the German Research Association (DFG, 2019-2025).
International consortium PAVE
‘A Nanovaccine Approach for the treatment of Pancreatic Cancer’ is an Integrated Training Network (ITN) funded by the European Union to develop peptide-based nanovaccines for pancreatic cancer (2020-2023).
T-Lock Consortium
This consortium unravels immune regulatory pathways in malignant melanoma, NSCLC and colorectal cancer (German Cancer Aid: 2019-2023)
The team are dedicated to developing innovative cell-therapy approaches for the treatment of patients with advanced cancer. Late-phase development projects include:
Antigen receptor transduced T cells with genetically engineered therapeutic payload
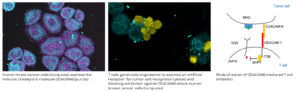
Immune checkpoint blockade by CEACAM6 inhibition is based on our identification of CEACAM6 as an important novel immune checkpoint molecule in many cancers. Together with the German Cancer Research Center we exploit a proprietary blocking antibody against this molecule which improves anti-cancer activity of tumor specific T cells. We have generated Antigen receptor transduced tumor specific T cells that produce and secrete this blocking antibody once they encounter a tumor cell so that their anti-tumor activity is improved. This concept is in preclinical development at the LIT.
Witzens-Harig et al. 2013, Blood;121(22):4493-503., Pinkert et al. 2022, Oncoimmunology;11(1):2008110
Inhibiting immune-resistance mechanisms
Our division has identified a novel immune resistance mechanism that is active in many cancers. It is based on the salt inducible kinase 3 which can transform a cytotoxic attack of a T cell into an activating, protumorigenic signal. Based on this finding, the spin off company iOmx —co-founded in 2016 by the division’s head Professor Beckhove— has developed a SIK3 inhibitor which has recently entered clinical testing.
Sorrentino et al., Journal for ImmunoTherapy of Cancer 2022; 10(5):e004258; Clinical Trials.gov Identifier: NCT05826600
Prof. Philipp Beckhove
Phone: +49 941 944 – 38101
E-Mail: beckhove@rcii.de