Prof. Luca Gattinoni
Head of Research Division | Functional Immune Cell Modulation

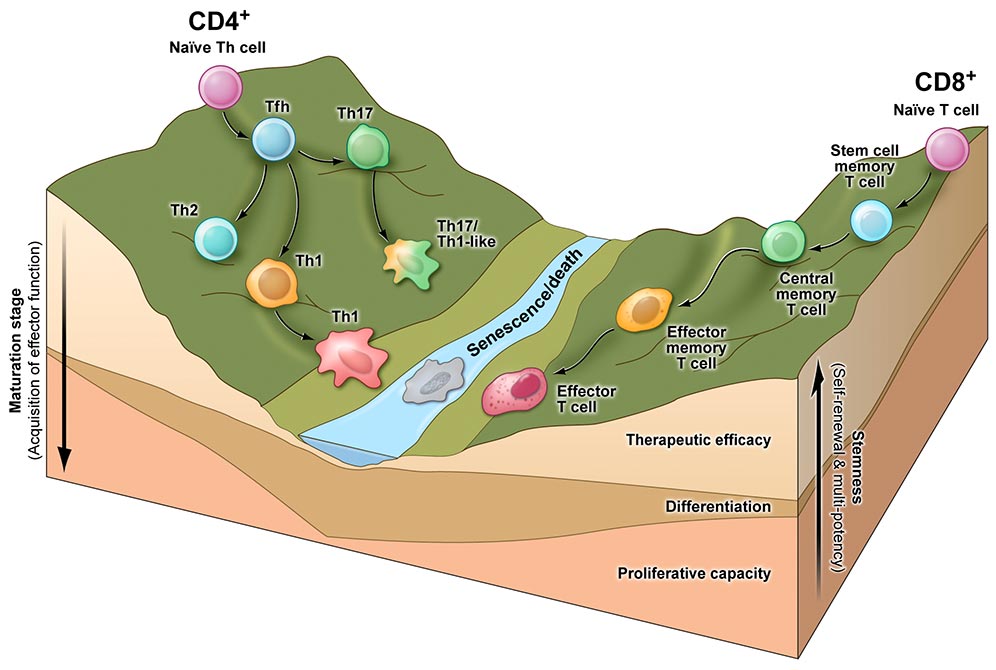
Professor Luca Gattinoni has been dedicated to developing curative T-cell-based immunotherapies in advanced cancer patients for over 20 years. Throughout his career a central theme of his research has been to elucidate how the differentiation state of T cells impacts their effectiveness in adoptive T-cell transfer for tumor eradication. His discoveries have fundamentally shaped the current view in the field which now recognizes the superior therapeutic potential of early memory T cells compared to cytotoxic effector T cells (Gattinoni L. et al, J Clin Invest, 2005 and Gattinoni L. et al, Nat Med, 2009).
Within this framework, Professor Gattinoni uncovered a unique subset of minimally differentiated memory T cells characterized by stem cell-like attributes, aptly named stem cell memory T cells (Tscm). And provided the first evidence that these cells mediate superior antitumor responses after adoptive transfer (Gattinoni L, et al. Nat. Med, 2011).
Recent work
In recent developments, Professor Gattinoni has broken new ground by establishing a clinical-grade manufacturing platform for the production of CAR-modified Tscm cells (Sabatino M, et al, Blood 2016) and by initiating a first-in-human study to test the safety and efficacy of donor-derived CD19-CAR Tscm cells (NCT01087294).
Professor Gattinoni’s current research endeavors are concentrated on reprogramming the fate and function of T cells. These strategic approaches encompass both pharmacologic and genetic interventions, targeting key transcription factors (Ji Y. et al, Nat Immunol, 2013; Gautam S, et al., Nat Immunol, 2019), epigenetic regulators (Ji Y. et al, Nat Commun, 2019), microRNAs (Ji Y. et al, Proc Natl Acad Sci USA, 2015), and metabolic pathways (Sukumar et al, J Clin Invest, 2013, and Hermans D, et al. Proc Natl Acad Sci USA, 2020). These strategies collectively aim to promote a stem cell-like behavior in T cells, offering promising avenues for advancing immunotherapies in the fight against cancer.
Quote from Prof. Luca Gattinoni
By enhancing T-cell stemness, our goal is to enable T cells to endure long-lasting battles against cancer stem cell-induced tumors.
Head of Research Division Functional Immune Cell Modulation
Biography

Academic background and qualifications
Prof. Gattinoni graduated from the University of Milan School of Medicine with distinction in 1998 and completed his residency in Medical Oncology at the National Cancer Institute of Milan (INT) in 2003. He received postdoctoral training in Cellular Immunotherapy and Tumor Immunology alongside Dr. Restifo at the Surgery Branch of the U.S. National Cancer Institute (NCI) part of the National Institute of Health (NIH) from 2003–2008.

Professional career
Prof. Gattinoni remained at the NCI until 2019 as Staff Scientist (2008–2013) and NIH Stadtman Investigator (2013-2019). Since 2019, he holds the Chair of Functional Immune Cell Modulation at the University of Regensburg, Germany. He is currently the Director of the Division of Functional Immune Cell Modulation at the LIT.

Honors
Prof. Gattinoni has published more than 100 manuscripts and has been the recipient of several prizes and honors including the SITC Presidential Award (2004), the Wilson S. Stone Memorial Award (2012), and the NCI Director’s Intramural Innovation Award (2013). Since 2023 he has been an honorary professor at the Amity Institute of Molecular Medicine and Stem Cell Research, Amity University, Noida, India.
Explore our Research Division in greater depth
Get to know our team and find out more about our pioneering research.
-
Hermans D, Gautam S, García-Cañaveras JC, Gromer D, Mitra S, Spolski R, Li P, Christensen SM, Nguyen R, Lin JX, Oh J, Du N, Veenbergen S, Fioravanti J, Ebina-Shibuya R, Bleck CKE, Neckers L, Rabinowitz JD, Gattinoni L, Leonard WJ. LDH inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor Immunity. Proc Natl Acad Sci USA (2020) 17:6047–6055.
-
Gautam S, Fioravanti J, Zhu W, Le Gall JB, Brohawn P, Lacey NE, Hu J, Hocker JD, Hawk NV, Kapoor V, Telford WG, Gurusamy D, Yu Z, Bhandoola A, Xue HH, Roychoudhuri R, Higgs BW, Restifo NP, Bender TP, Ji Y, Gattinoni L. The transcription factor c-Myb regulates CD8+ T cell stemness and antitumor immunity. Nat Immunol (2019) 20:337–349
-
Ji Y, Fioravanti J, Zhu W, Wang H, Wu T, Hu J, Lacey NE, Gautam S, Le Gall J, Yang X, Hocker JD, Escobar TM, He S, Dell’Orso S, Hawk NV, Kapoor V, Telford WG, Di Croce L, Muljo SA, Zhang Y, Sartorelli V, Gattinoni L. miR-155 harnesses Phf19 to potentiate cancer immunotherapy through epigenetic reprogramming of T cell fate. Nat Commun (2019) 10:2157
-
Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med (2017) 23:18–27
-
Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, Dougherty S, Qin H, Klebanoff CA, Fry TJ, Gress RE, Kochenderfer JN, Stroncek DF, Ji Y, Gattinoni L. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood (2016) 128:519–528
-
Ji Y, Wrzesinski C, Yu Z, Hu J, Gautam S, Hawk NV, Telford WG, Palmer DC, Franco Z, Sukumar M, Clever D, Roychoudhuri R, Klebanoff CA, Surh CD, Waldmann, TA, Restifo, NP, Gattinoni L. miR-155 augments CD8+ T cell anti-tumor activity in lymphoreplete hosts by enhancing responsiveness to homeostatic γc cytokines. Proc Natl Acad Sci USA (2015) 112:476–481
-
Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, Gattinoni L. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and anti-tumor function. J Clin Invest (2013) 123:4479–4488
-
Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick, E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human T cell memory subset with stem cell-like properties. Nat Med (2011)17:1290–1297
-
Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, Wang E, Schrump DS, Marincola FM, Restifo NP, Gattinoni L. Repression of the DNA-binding inhibitor Id3 by Blimp1 limits the formation of memory CD8+ T cells. Nat Immunol (2011)12:1230–1237
-
Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Church L, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med (2009)15:808–813
Many thanks to the funding agencies who support our work:
2022–2027: German Research Foundation (DFG), Reinhart Koselleck Project
Reprogramming CD8+ T-cell metabolism and fate by MSC mitochondrial transfer.
2022–2026: EIC Pathfinder Challenges 2021
Fine-tuning T cell networks of exhaustion by synthetic sensors.
2021–2025: German Research Foundation (DFG), CRC/ TR221, Project A07
Enhancing graft-versus-leukemia responses by donor-derived CAR-modified CD8+ T memory stem cells.
2021–2025: EIC Pathfinder
Immune niches for cancer immunotherapy enhancement.
We participate in several national and international research consortia:
EIC-Pathfinder T-FITNESS: Fine-Tuning Networks of Exhaustion by Synthetic Sensors
This EU consortium aims to generate antitumor T cells which are refractory to terminal differentiation and dysfunction. This ambitious goal will be pursued through the development of innovative microRNA-based synthetic logic circuits capable of rewiring the transcriptional programs driving T-cell exhaustion. The leadership and coordination of this project are centered at the LIT, where our primary focus lies in comprehensively characterizing the phenotype, function, and metabolic profiles of T cells that have undergone reprogramming using T-FITNESS technology.
https://www.t-fitness-horizon.eu/
EIC Pathfinder INCITE: Immune Niches for Cancer Immunotherapy Enhancement
This EU consortium seeks to develop a 3D-printed immune niche microenvironment for the generation and expansion of tumor-specific stem cell memory T cells. Within this consortium, we are responsible for functionally evaluating human CAR T cells generated within INCITE artificial immune niches and comparing them with those manufactured with the current technology.
CRC/ TR221 – GvHGvL
The focus of this national consortium is to improve the safety and efficacy of allogeneic stem cell transplantation by developing innovative strategies to modulate graft-versus-host and graft-versus-leukemia immune responses. Our role in this Collaborative Research Center is to investigate whether donor-derived CAR-modified CD8+ T memory stem cells provide a safer and more effective platform to treat human B-cell malignancies which have relapsed after allogeneic stem cell transplantation.
We are dedicated to developing innovative cell-therapy platforms for the treatment of patients with advanced cancer. Late-phase development projects include:
Collaboration with Miltenyi Biotec
We are developing a cGMP-compliant, semi-automated manufacturing platform for the generation of CD22/CD19-bispecific CD8+ Tscm cells. The cellular product developed will be tested in patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma.
Collaboration with Poseida Therapeutics
We are developing a manufacturing platform for the generation of NY-ESO-1-TCR redirected Tscm cells, functionally empowered through the overexpression of human miR-155 SNP rs377265631. The cellular product developed will be tested in patients with relapsed or refractory sarcoma.
Prof. Luca Gattinoni
Tel: +49 941 944–38131
Email: luca.gattinoni@lit.eu